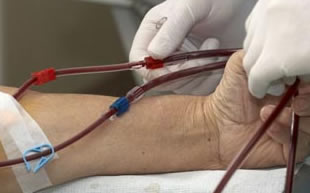
The biochemistry of a living creature is a delicate balancing act. For example, the pH level of the blood – the balance of acidity and alkalinity must be just right in order for a person to remain in good health. Too much acidity in the blood results in acidosis, a condition that can cause an irregular heartbeat as well as an elevated hear rate. When the blood is too alkaline, it causes alkalosis, which may result in muscle spasms and cramps.
Normally, the kidneys assist in regulating this balance. However, for patients with kidney disease and who must undergo regular dialysis as part of their treatment, medication during the process is necessary in order to preserve the proper pH levels.
It is a “medical device”, a dry-powdered acid concentrate, that is at the heart of the GranuFlo litigation. Allegedly, German pharmaceutical firm Fresenius, which manufactures and markets the dialysis products drugs GranuFlo and NaturaLyte, failed to issue proper warnings to physicians, including those outside of its own network of clinics, about possible complications related to their products. These complications are allegedly responsible for dialysis patients suffering heart attacks, stroke and even death.
As of this writing, the number of plaintiffs who have each filed a GranuFlo lawsuit numbers just under 5,500. Lauren Barnes, a Massachusetts litigator representing a plaintiff in a GranuFlo lawsuit, was quoted in the Boston Herald as stating that Fresenius “…turned a blind eye to the injuries the products could and would cause…[and] allowed thousands of individuals and their families to suffer significantly and needlessly.”
Despite this, Fresenius continues to defend its products, which, despite a Class 1 Recall from the U.S. Food and Drug Administration in June of 2012, remain on the market. Kent Jarnell, a spokesman for the company, told the Herald that his company’s products are “are safe and effective when used in accordance with their labels and instructions for use.”
A bellwether trials are being scheduled for late next later this year in order to determine Fresenius liability.