Recent efforts to reach a settlement agreement in the Cook IVC Filter Litigation were unsuccessful, meaning a select number of cases for early “bellwether” trials could get underway next year, if additional settlement attempts don’t result in an agreement.
Over 500 plaintiffs have joined the Cook IVC Filter Litigation since October 2014, when the U.S. Judicial Panel on Multidistrict Litigation consolidated all federally filed Cook IVC filter lawsuits into one court in the Southern District of Indiana. The plaintiffs claim they were injured by Cook Celect and Gunther Tulip IVC filters, medical devices designed to capture a blood clot and prevent it from entering the lungs.
In June, attorneys for both sides met to discuss potential settlement agreements with U.S. Magistrate Judge Tim Baker, but no resolution was reached. There could be other attempts, but if attorneys can’t agree on a settlement, the bellwether cases will be tried before juries.
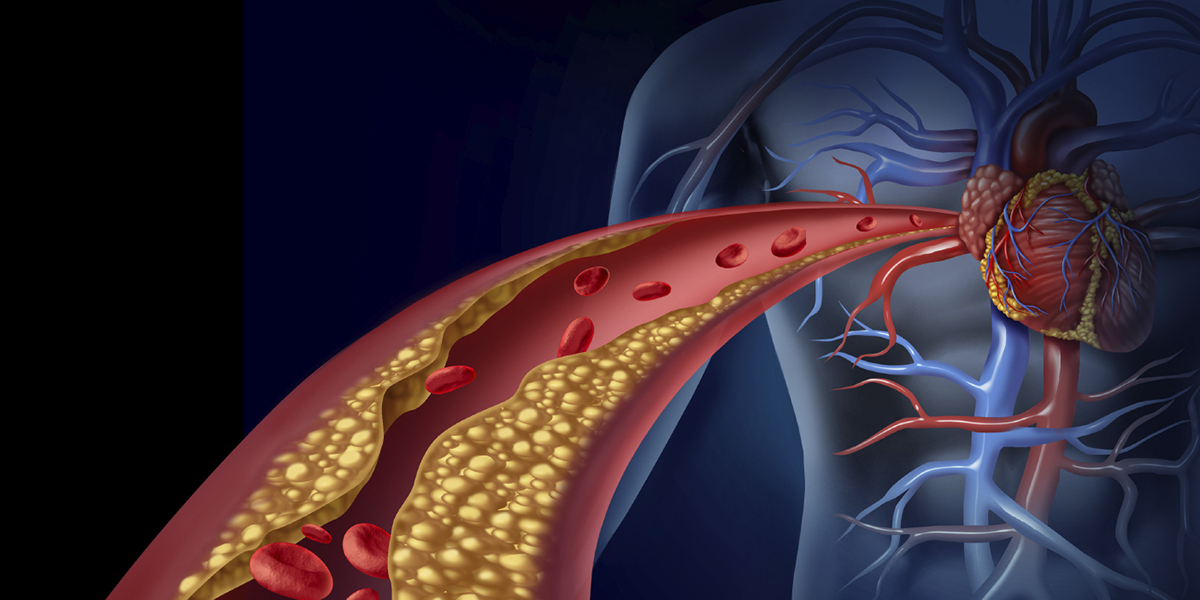
Cook IVC filers are small, cage-like devices implanted in the main vein traveling from the legs back to the lungs and heart. Blood clots can move through this vein and cause a life-threatening embolism. The filters are designed are to capture them and render the clots harmless, but the devices can break, fracture, and even migrate to other organs and parts of the body where they can cause blood clots and other health problems.
“One study showed that 40% of a particular brand of IVC filters fractured after five a half years,” said Jeff Gaddy, an attorney with the Levin, Papantonio law firm in Pensacola. “When the leg of a filter fractures, it is susceptible to travel to up the bloodstream and potentially puncture an organ or another vital part of the body.”
Between 2005 and 2010, nearly 1,000 adverse events were reported to the FDA related to IVC filters, with over 300 cases of the device migrating to the heart of pulmonary artery, causing a number of medical problems including severe injuries and death. In May 2014, the FDA advised doctors to remove IVC filters with a month to two months of implantation, as long as the danger of pulmonary embolism has passed.
“Despite that communication from the FDA, the drug companies that manufacture IVC filters, continue to tell physicians that removal of the devices is optional,” said Gaddy.
The early bellwether trails are scheduled to begin in 2017. Depending on how those early cases conclude, the two sides may be better able to come to a settlement agreement. If not, all pending cases will be remanded back to their original courts of filing for individual trials.