Last year, the European Medicines Agency (EMA) started an investigation into the possibility that use of the diabetic medication Invokana (canagliflozin) increased a patient’s risk of limb amputation. Although solid evidence of this risk had not yet been found, the EMA chose to err on the side of caution, requiring packages in the EU to carry a warning to that effect.
Similar clinical studies were underway in the US, but the Food and Drug Administration as usual refused to go beyond issuing an “advisory.” This week, researchers came to a definite conclusion: Invokana definitely increases the risk that a diabetic patient will lose a lower limb to the disease.
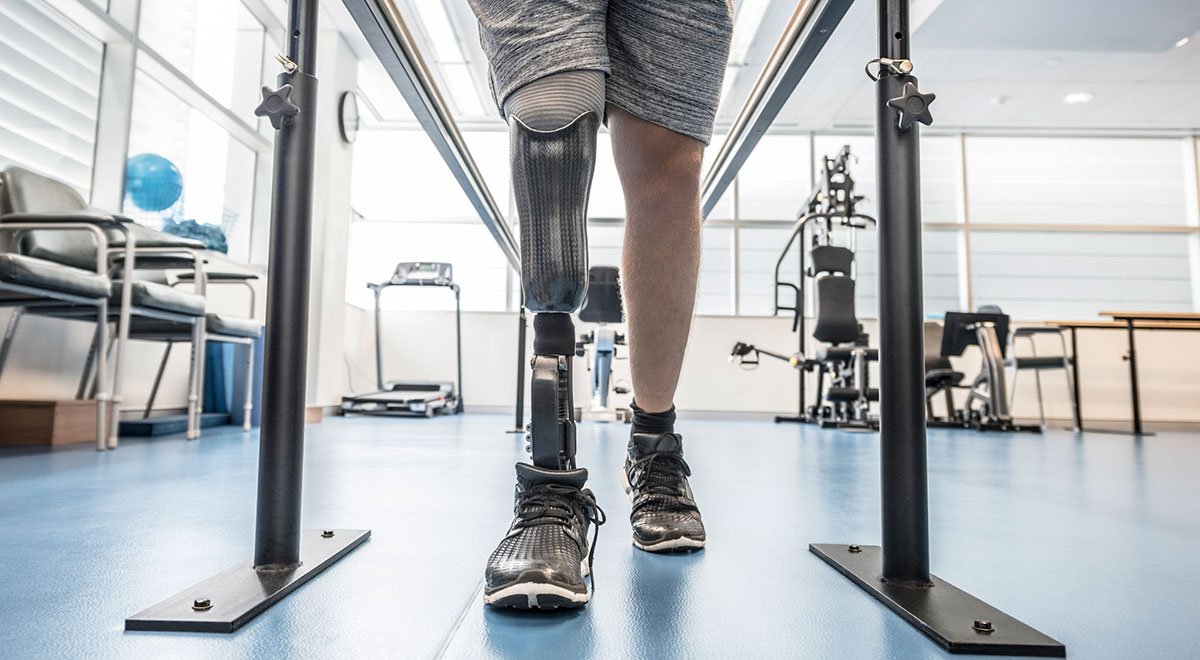
The final results from two studies (CANVAS and CANVAS-R) showed that patients taking Invokana wound up having to have foot and leg amputations twice as often as those who were given a placebo. According to the CANVAS study, nearly six out of every 1,000 Invokana patients will suffer complications requiring limb amputation over a twelve-month period. Results for subjects participating in the CANVAS-R were even more grim: the rate of amputation for this group was 7.5 per 1,000.
Most amputation involved the loss of toes and the middle of the foot, but leg amputations also occurred. Some patients wound up losing both legs.
Invokana packaging in the US will now be required to carry a “black box” warning. Physicians are advised to have patients discontinue the use of Invokana if they begin to experience limb pain, or develop sores, ulcers or infections of the leg or foot. Patients should not take Invokana if they have a history of prior amputations, suffer vascular disease or have experienced diabetic neuropathy (nerve damage).
Ironically, canagliflozin – a sodium-glucose cotransporter-2 (SGLT-2) inhibitor – was intended to prevent many of the medical problems to which it has now been linked – including kidney damage, a common risk of diabetes. Respected medical professionals raised concerns about Invokana even before drug maker Janssen (a subsidiary of Johnson & Johnson, a defendant in numerous lawsuits) began seeking FDA approval for the product. However, the FDA, bowing to the wishes of the manufacturers, determined that the benefits outweighed the risks and gave its approval – on the condition that Janssen would conduct its own studies and monitoring for adverse events.
Plaintiffs in current lawsuits against Janssen and Johnson & Johnson now allege that the companies were fully aware of the dangerous side effects of Invokana, which also include ketoacidosis, stroke and cardiac arrest, and deliberately withheld this information from the public.