Only months after medical device manufacturer C.R. Bard agreed to pay out $200 million in settlements over its vaginal mesh product, the New Jersey-based company is facing more legal problems over a defective medical device. This time, however, the company’s behavior has allegedly crossed the line from negligence to criminal fraud.
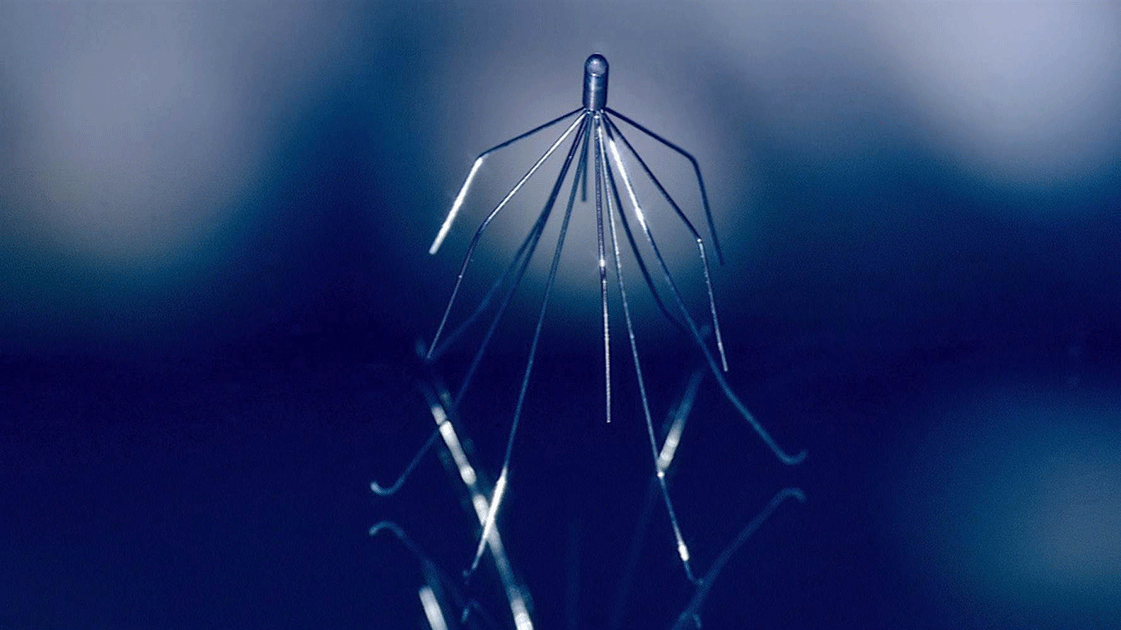
The product at the center of the most recent litigation is the “Inferior Vena Cava,” or IVC filter. Resembling a common cellar spider (better known as the “daddy longlegs”), this device is designed for implantation into the major vein that carries deoxygenated blood from the lower body into the right atrium, or collection chamber, of the heart. Its purpose is to prevent pulmonary embolism, or blockage of the flow of blood from a clot. Despite serious questions about the IVC filter’s safety and effectiveness as well as recommendations that they be used only for high-risk cases, a quarter-million such devices are implanted in patients annually.
Recently, NBC News uncovered a report dated December 15, 2004, from Dr. John Lehmann, a physician hired by C.R. Bard to conduct an independent study of the Recovery filter. Dr. Lehmann raised grave concerns about the device. He found that the device was more dangerous than any similar filter then on the market, concluding that “further investigation of the filter in relation to migration and fracture is urgently warranted.”
Over the next six years, the Food and Drug Administration (FDA) received more than 900 reports of adverse events associated with the Recovery IVC Filter, including fracture and migration. Small metal parts are known to become lodged in veins, causing perforation and death. Nearly 30 fatalities have been attributed to Bard’s IVC filter.
In light of Dr. Lehmann’s report and warnings, why did Bard continue with the manufacture and sale of the IVC filter? The explanation is all-too-common: in order to preserve its profits. Bard executives realized that a redesign was in order, but the new model would not be ready for at least a year. Instead of pulling the earlier model, Bard decided not to submit the required report to the FDA – putting the company in violation of federal law.
The new model wasn’t much better. At this point, the story gets even worse: when a regulatory specialist for Bard refused to sign off on an FDA application for approval of the device, someone at the company simply forged her signature on the document.
Since the new model was released in 2005, there have been well over 900 reports of adverse events due to the IVC filter. In 2010, a study published in the Archives of Internal Medicinefound that 16% of the IVC filters implanted in patients had at least one fractured strut. The researchers concluded that “The Bard Recovery and Bard G2 filters had high prevalence of fracture and embolization, with potentially life-threatening sequelae [consequences].”
Bard’s initial response was to hire publicists in order to shore up the company’s public image. Since plaintiffs started coming forward with lawsuits, Bard has filed numerous motions to have the actions dismissed. So far, it has done the company little good. Last week,Reuters reported that litigation against C.R. Bard, such as that being handled by mass torts law firm Levin Papantonio, was among the fastest growing during the month of October. This past summer, the FDA sent a warning letter to the company, citing numerous violations of federal law, including the sale of “adulterated” and “misbranded” products, failure to report adverse events, and withholding safety information from consumers.