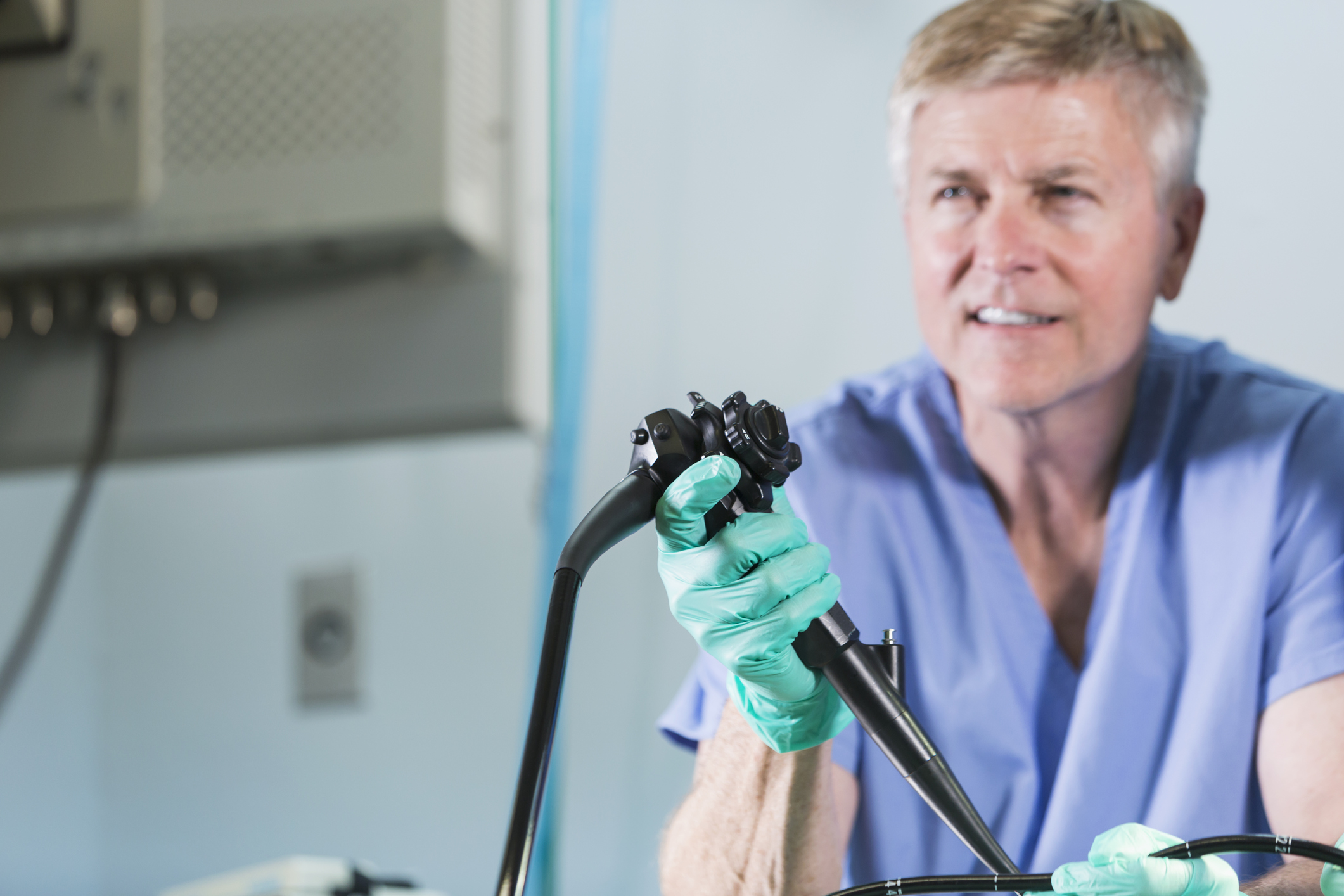
Olympus, the manufacturer of endoscopes that have been implicated in serious infections and at least 35 patient deaths, has announced a partnership with Ruhof Healthcare, a company specializing in surgical and medical decontamination products.
Beginning this month, Olympus will serve as the exclusive U.S. distributor of Ruhof’s Guardian Endoscope Single-Use Valve Set. As the name implies, these valves are disposable. It is hoped they will address the problem of cross-contamination that has led to the spread of antibiotic-resistant bacteria.
The problem of patient infections stemmed from a design flaw that made Olympus scopes difficult to properly clean and sterilize. Tokyo-based Olympus began receiving reports of these infections from European hospitals in 2012. The company issued an alert in the E.U., but according to a report published in the Los Angeles Times, Olympus executives in the U.S. were instructed not to issue similar warnings in this country.
Olympus is now facing numerous lawsuits as well as a possible criminal investigation. Last summer, a jury awarded $1 million to the widow of a patient who died as the result of an infection contracted from a contaminated Olympus duodenoscope, as well as $6.6 million to the Seattle hospital where the procedure was performed.
In a company press release announcing the new distribution deal with Ruhof, Olympus America Inc. executive Kurt Heine said, “The addition of this innovative solution to our EndoTherapy portfolio allows us to further our commitment to patient safety by eliminating the need for reusable valves.”
Ruhof’s Vice President of Sales and Marketing Douglas MacKay added that his company is “enthusiastic about working with Olympus…this collaboration will further our efforts to help healthcare professionals meet and exceed their decontamination challenges while ensuring patient safety.”
Ruhof is not the only company to offer disposable endoscope valves. Medivators, a division of New Jersey-based Cantel Medical Corporation, now offers its own series of sterile single-use valves for both Olympus and Pentax endoscope products.
The new partnership with Ruhof is only one of a number of such deals being made with other companies as Olympus attempts to expand its product line. In addition, Olympus is the exclusive distributor for a set of surgical knives used during endoscopic examinations, manufactured by Sumitomo Bakelite Ltd., and is entering into a marketing and promotion agreement with Aries Pharmaceuticals for its Eleview, a product used in the removal of polyps in a patient’s colon.
Heine says,
“With each device we unveil to the GI community, Olympus gains strength as being more than an excellent imaging partner; we evolve into a complete solutions provider to our customers. Strategic partnerships, a commitment to innovation and a drive to provide methods to raise quality and satisfaction while lowering costs – these are ways we build trust with our customers every day.”
Between the problems Olympus has had with their endoscopes, their failure to warn the U.S. medical community, the lawsuits that are being filed as a result of that failure and a past record of criminal behavior, the company will have to work very hard to earn that trust going forward.