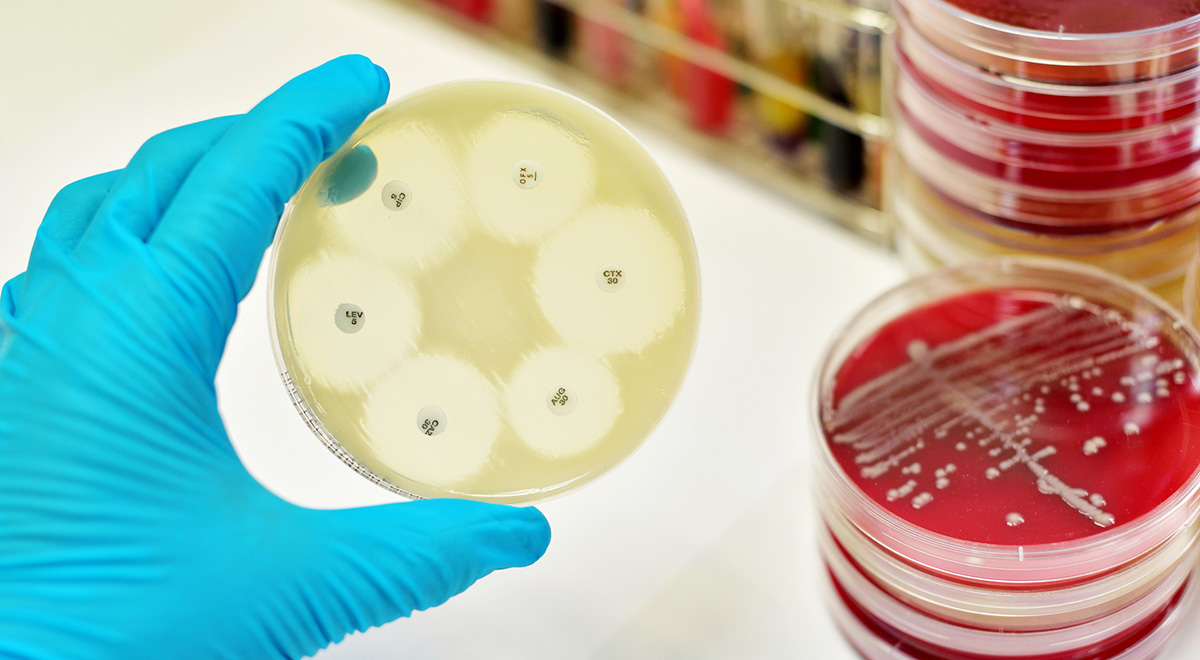
Overuse of antibiotics over the past several decades has led to the evolution of drug-resistant “superbugs,” which has serious consequences for human health in the coming years. A report from the Centers for Disease Control indicates that antibiotic-resistant pathogens are responsible for 23,000 deaths in the U.S. annually. If this trend continues, antimicrobial resistance (AMR) could kill as many as ten million people globally by mid-century, and cost the world’s economy as much as $100 trillion per year.
There is one ray of hope, however; it turns out that as these bacteria develop resistance to antibiotics, they either require greater amounts of nutrients, or they become less efficient at acquiring them. This fact has led to a new line of research into the question of whether superbugs could simply be starved into submission.
In a recent research study, scientists were able to essentially turn drug-sensitive bacteria against drug-resistant superbugs, forcing the latter to compete with the former for nutrients and resources. It turns out that pathogens that do respond to antibiotics are essentially better at getting food than superbugs. According to biologist Andrew Read, one of the co-authors of the study, the work involved “…utilizing the natural force of competition to control the resistant ones and using conventional drugs to treat the sensitive ones.”
In the experiments, the research team treated drinking water that was given to malaria-infected laboratory mice, changing the amount of a nutrient that feeds the bacteria. The mice were given the standard treatment for the disease, which had a 40% failure rate – an indication of AMR. Next, the amount of the malaria nutrient in the water was reduced significantly. Afterward, the mice began responding to the treatment, and the infection did not return.
Lead author Nina Wale says, “By limiting this nutrient, we prevented the emergence of drug resistance.”
The next step was to determine whether the results were due to competition among the parasites or some other factor related to limiting the nutrient. Mice infected with resistant malaria in the presence of limited nutrients did not respond to treatment. However, when a drug-sensitive strain was introduced, the resistant parasites failed to thrive.
Essentially, the introduction of drug-sensitive bacteria caused the resistant strain to starve.
For years, pharmaceutical research has been focused on a virtual “arms race,” trying to stay one step ahead of bacterial evolution by developing newer, more powerful antibiotics – which in turn, invariably leads to greater antimicrobial resistance. The results of this study suggest that such an “arms race” may be unnecessary, giving hope that existing treatments for bacterial infection can continue to be effective. Read points out that their work “…is a proof-of-principle that an ecological manipulation can make it possible to continue using a drug, even when resistant pathogens that would otherwise cause a treatment failure are present at great numbers.”
Read adds that adding resource-limiting intervention into the research and development of new treatments could increase those costs in the short term – but it would soon pay for itself. Considering that bringing new medications to market can cost upward of $100 million, introducing resource limitation into the process could extend the lifespan of existing treatments. “The payoff could be quite big,” he says.