
Welcome to Drug Safety News. It’s our goal to provide you current news stories and videos regarding various prescription drugs and medical devices that have been the subject of recalls and/or national lawsuits. Just because a prescription drug or medical device is discussed below does not mean it’s causing you harm. It simply means that safety issues have been raised.
You should not stop using your medication or device simply because it’s written about. Instead, you should click on the story, and become informed of the potential issues. If you have concerns after reading the information, then you should consult your physician, and determine the proper medical course for you.
FEATURED DRUGS AND DEVICES
FEATURED NEWS
A Venerable Vaccine May Represent New Hope For Type 1 Diabetics
Type 1, also known as “childhood onset” diabetes, is a genetic autoimmune disorder in which the body’s own defenses turn on cells in the pancreas, rendering it non-functional. Its function, of course, is to produce insulin, the hormone that transports and metabolizes glucose. People with this condition depend on regular insulin injections in order to


Failing to Reach a Settlement Abilify Bellwether Cases Head to Trial
Since the passing of a recent deadline to reach a global settlement in nearly 1,700 Abilify lawsuits (up from the 22 cases originally consolidated in 2016), counsel for defendants Otsuka Pharmaceuticals and Bristol-Meyer Squibb (BSM) and those for the plaintiffs have...
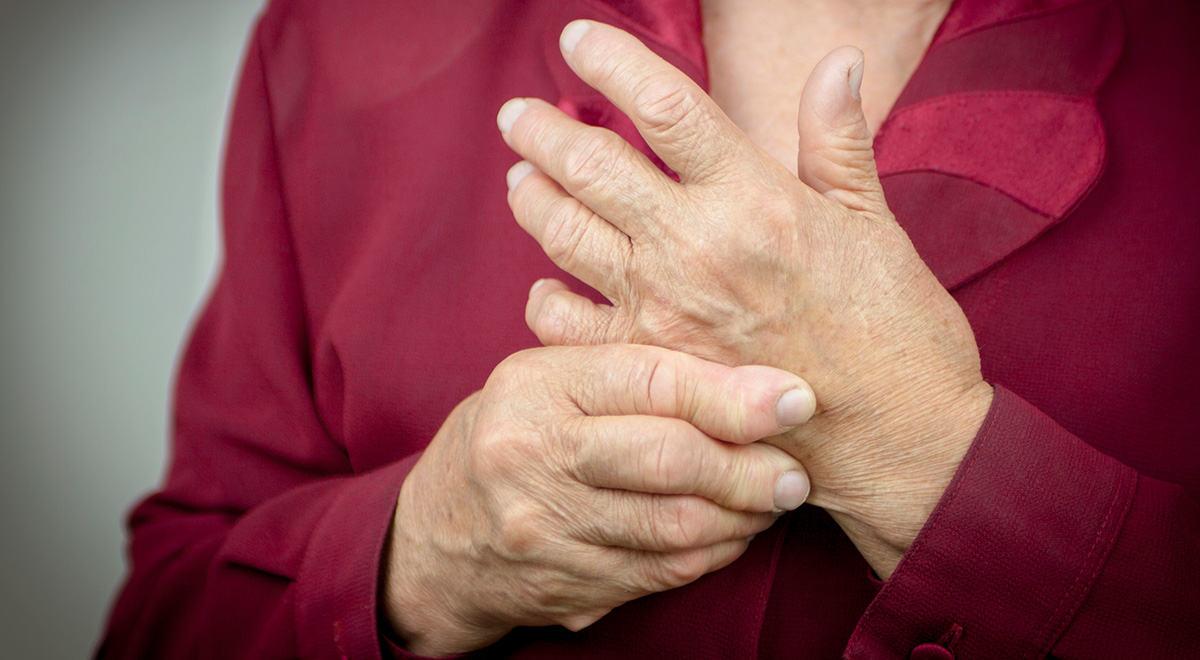
FDA Refuses to Approve Actemra Competitor, Citing Concerns Over Heart Attacks – Yet Actemra Carries No Such Warnings
One of the major problems with drugs used to treat rheumatoid arthritis is the side effects that can cause heart attacks, stroke, respiratory infections and other serious conditions. Supposedly, Actemra (tocilzumab), a product of Roche and Genentech, didn't cause...

Defective INRatio Monitor System Resulting in Severe Injuries
By now, the allegations against Alere over its INRatio and INRatio 2PT/INR Monitor System and INRatio Test Strips are well known to the legal community and patients who have used these devices. Those patients are ones treated with the anti-coagulant drug warfarin or...

Attune Knee Replacement Failures and Consequences
A recent study published in the Journal of Knee Surgery reviewed a number of reports submitted to the FDA's Manufacturer and User Facility Device Experience (MAUDE) database regarding DePuy Orthopaedic's Attune Knee System, and found more than 230 reports about device...
CURRENT NEWS

Failing to Reach a Settlement Abilify Bellwether Cases Head to Trial
Since the passing of a recent deadline to reach a global settlement in nearly 1,700 Abilify lawsuits (up from the 22 cases originally consolidated in 2016), counsel for defendants Otsuka Pharmaceuticals and Bristol-Meyer Squibb (BSM) and those for the plaintiffs have...

Deadline for Abilify Makers Less Than a Month Away
As of April of 2018, the were over 800 lawsuits pending against Otsuka Pharmaceuticals and its U.S. partner, Bristol-Meyer Squibb (BMS) over the psychoactive medication Abilify (aripiprizole). Plaintiffs allege that the drugmakers failed to warn patients and doctors...

Abililfy Manufacturers Are Off the Hook in Whistleblower Lawsuit
A six-year legal battle has finally ended for drugmakers Bristol-Meyer Squibb (BMS) and Otsuka as a judicial panel recently made a 2 to 1 decision not to revive a whistleblower lawsuit filed by two former employees. In U.S. v. Bristol Meyers Squibb Co., the Sixth...






